Autoinductive conversion of α,α-diiodonitroalkanes to amides and esters catalysed by iodine byproducts under O2†
Abstract
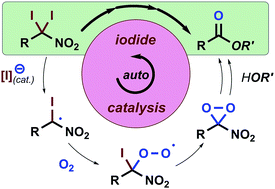
Studies to convert nitroalkanes into amides and esters using I2 and O2 revealed in situ-generated iodine species facilitate the homolytic C–I bond cleavage of α,α-diiodonitroalkanes, arguably in an autoinductive or autocatalytic manner. Consequently, we devised a rapid and economical I2/O2-based method to synthesise sterically hindered esters directly from primary nitroalkanes.


 Please wait while we load your content...
Please wait while we load your content...