Synthesis of tetronic acids from propargylic alcohols and CO2†
Abstract
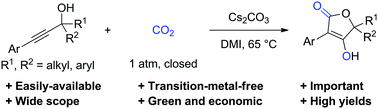
A direct and practical synthesis of important tetronic acids from easily available propargylic alcohols and carbon dioxide is reported for the first time. This transition-metal-free transformation features high atom- and step-economy, mild reaction conditions, good functional group tolerance and high yield. Preliminary mechanistic studies suggest that the reaction proceeds via cyclization to give alkylidene cyclic carbonate, ring-opening and re-cyclization processes.


 Please wait while we load your content...
Please wait while we load your content...