Highly-active oxygen evolution electrocatalyzed by an Fe-doped NiCr2O4 nanoparticle film†
Abstract
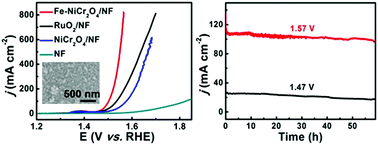
Alkaline water splitting offers a simple method for the mass production of hydrogen but suffers from the sluggish kinetics of the anodic oxygen evolution reaction (OER). Here, we report on the development of an Fe-doped NiCr2O4 nanoparticle film on Ni foam (Fe–NiCr2O4/NF) as a non-noble-metal OER electrocatalyst with superior catalytic activity at alkaline pH. Such Fe–NiCr2O4/NF demands overpotentials as low as 228 and 318 mV to drive current densities of 20 and 500 mA cm−2, respectively, in 1.0 M KOH. Notably, it also shows strong long-term electrochemical durability with its activity being retained for at least 60 h.


 Please wait while we load your content...
Please wait while we load your content...