The ortho-substituent on 2,4-bis(trifluoromethyl)phenylboronic acid catalyzed dehydrative condensation between carboxylic acids and amines†
Abstract
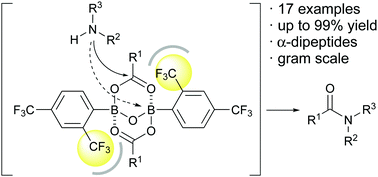
2,4-Bis(trifluoromethyl)phenylboronic acid is a highly effective catalyst for dehydrative amidation between carboxylic acids and amines. Mechanistic studies suggest that a 2 : 2 mixed anhydride is expected to be the only active species, and the ortho-substituent of boronic acid plays a key role in preventing the coordination of amines to the boron atom of the active species, thus accelerating the amidation. This catalyst works for α-dipeptide synthesis.


 Please wait while we load your content...
Please wait while we load your content...