Aβ under stress: the effects of acidosis, Cu2+-binding, and oxidation on amyloid β-peptide dimers†
Abstract
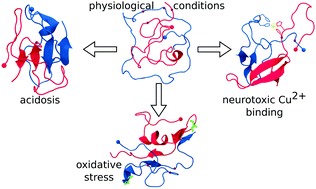
In light of the high affinity of Cu2+ for Alzheimer's Aβ1–42 and its ability to subsequently catalyze the formation of radicals, we examine the effects of Cu2+ binding, Aβ oxidation, and an acidic environment on the conformational dynamics of the smallest Aβ1–42 oligomer, the Aβ1–42 dimer. Transition networks calculated from Hamiltonian replica exchange molecular dynamics (H-REMD) simulations reveal that the decreased pH considerably increased the β-sheet content, whereas Cu2+ binding increased the exposed hydrophobic surface area, both of which can contribute to an increased oligomerization propensity and toxicity.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...