An umpolung of Lewis acidity/basicity at nitrogen by deprotonation of a cyclic (amino)(aryl)nitrenium cation†
Abstract
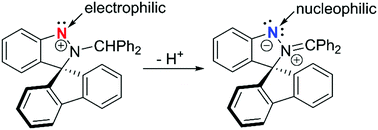
A cyclic (amino)(aryl)nitrenium cation 2 has been achieved by treatment of spiro[fluorene-9,3′-indazole] (1) with Ph2CHCl and AgBF4. This cation 2 is Lewis acidic at nitrenium N1, reacting with PMe3 affording a Lewis acid/base adduct 3. In contrast, deprotonation of 2 with other bases provides a neutral compound 4 which is Lewis basic at N1, reacting with electrophiles including GaCl3, MeOTf and PhNCO.


 Please wait while we load your content...
Please wait while we load your content...