Stereoselective synthesis of α-methyl and α-alkyl ketones from esters and alkenes via cyclopropanol intermediates†
Abstract
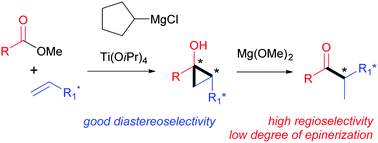
Alkenes bearing a stereocenter in the allylic position were found to undergo Kulinkovich hydroxycyclopropanation with good diastereoselectivity. For the isomerization of the resulting cyclopropanols to diastereomerically enriched α-methyl ketones, a new mild regioselective method has been developed. A sequence of stereoselective cyclopropanation and cyclopropanol ring opening was successfully employed for the construction of the C20 stereocenter in steroids.


 Please wait while we load your content...
Please wait while we load your content...