Rechargeable aluminum batteries utilizing a chloroaluminate inorganic ionic liquid electrolyte†
Abstract
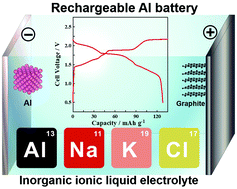
Rechargeable aluminum batteries composed of an aluminum anode, an expanded graphite cathode, and an inorganic chloroaluminate ionic liquid electrolyte show remarkably improved capacity, reversibility, and rate capability at 393 K compared to cells based on a common organic salt based ionic liquid, AlCl3–1-ethyl-3-methylimidazolium chloride.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...
