Capture of SO3 isomers in the oxidation of sulfur monoxide with molecular oxygen†
Abstract
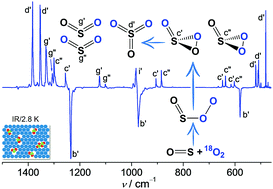
When mixing SO with O2 in N2, Ne, or Ar, an end-on complex OS–OO forms in the gas phase and can subsequently be trapped at cryogenic temperatures (2.8–15.0 K). Upon infrared light irradiation, OS–OO converts to SO3 and SO2 + O with the concomitant formation of a rare 1,2,3-dioxathiirane 2-oxide, i.e., cyclic OS(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(
O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 16O)18O and 16OS(
16O)18O and 16OS(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 18O)18O. The characterization of OS–OO and OS(
18O)18O. The characterization of OS–OO and OS(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
- This article is part of the themed collection: SU 120: Celebrating 120 Years of Soochow University


 Please wait while we load your content...
Please wait while we load your content...