Diverse ring opening of thietanes and other cyclic sulfides: an electrophilic aryne activation approach†
Abstract
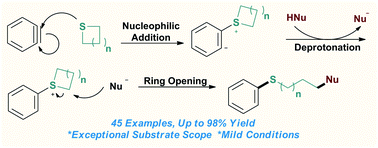
Organosulfides are a common class of structure units in bioactive molecules and functional materials motivating continuous developments of efficient synthetic methods. Herein, we report an electrophilic aryne-activated ring opening protocol of one or two heteroatom containing saturated sulfur heterocycles. This three-component transformation proceeds under mild reaction conditions and displays exceptional generality of nucleophiles (C, O, S, N, and F centered nucleophiles), giving structurally diverse thioethers in good yields.


 Please wait while we load your content...
Please wait while we load your content...