Organocatalytic asymmetric synthesis of 2,4-disubstituted imidazolidines via domino addition-aza-Michael reaction†‡
Abstract
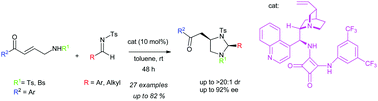
The first highly diastereo- and enantioselective synthesis of 2,4-disubstituted imidazolidines has been developed via a formal [3+2] cyclization reaction. Bidentate aminomethyl enones and N-tosyl imines were used as the reaction partners in the reaction. Bifunctional squaramide catalysts were found to be efficient for this reaction and few transformations of the products have been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...