Asymmetric formal synthesis of (+)-cycloclavine†
Abstract
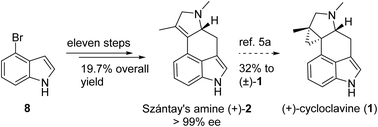
The asymmetric synthesis of Szántay's amine (+)-2, the pivotal precursor for direct access to (+)-cycloclavine (1), is described for the first time in eleven steps with 19.7% overall yield from the commercially available 4-bromoindole. The strategy features an asymmetric induction by Ellman's sulfinimine and rhodium-catalysed isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.


 Please wait while we load your content...
Please wait while we load your content...