Effect of buffer at nanoscale molecular recognition interfaces – electrostatic binding of biological polyanions†
Abstract
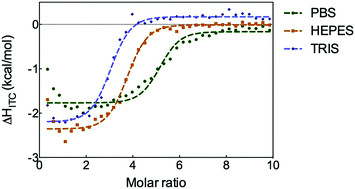
We investigate the impact of an over-looked component on molecular recognition in water–buffer. The binding of a cationic dye to biological polyanion heparin is shown by isothermal calorimetry to depend on buffer (Tris–HCl > HEPES > PBS). The heparin binding of self-assembled multivalent (SAMul) cationic micelles is even more buffer dependent. Multivalent electrostatic molecular recognition is buffer dependent as a result of competitive interactions between the cationic binding interface and anions present in the buffer.


 Please wait while we load your content...
Please wait while we load your content...