Fluorogenic diazaborine formation of semicarbazide with designed coumarin derivatives†
Abstract
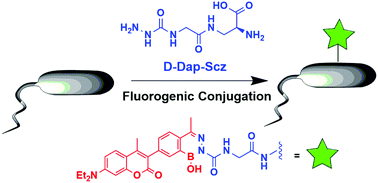
Bioorthogonal fluorogenic reactions serve as enabling tools in research and biotechnology. Herein we describe fluorogenic conjugations of semicarbazide with coumarin derivatives that incorporate a 2-acetylphenylboronic acid motif. These designed coumarins rapidly conjugate with semicarbazide to give diazaborine products with significantly enhanced fluorescence. To demonstrate potential applications of this fluorogenic reaction, we synthesized a semicarbazide-presenting amino acid D-Dap-Scz, which readily incorporates into the cell wall of Staphalococcus aureus and serves as a handle for conjugation with the coumarins. The fluorogenic conjugation of the coumarins to cell surface semicarbazide enables facile visualization of D-Dap-Scz treated bacteria.


 Please wait while we load your content...
Please wait while we load your content...