Rhodium-catalyzed odorless synthesis of diaryl sulfides from borylarenes and S-aryl thiosulfonates†
Abstract
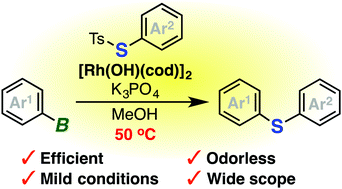
Various diaryl sulfides, including heteroaryl- and nitrogen-containing sulfides, have been efficiently prepared by rhodium-catalyzed odorless deborylative arylthiolation of organoborons with S-aryl thiosulfonates. The ready availability of starting materials and further transformation of sulfides have rendered a diverse range of organosulfur compounds easily accessible.


 Please wait while we load your content...
Please wait while we load your content...