Nickel-catalyzed direct C–H trifluoroethylation of heteroarenes with trifluoroethyl iodide†
Abstract
A highly selective nickel-catalyzed C–H trifluoroethylation of heteroarenes was developed with the assistance of a monodentate directing group. This protocol provides efficient access to various trifluoroethyl-substituted heteroarenes, including indoles, pyrroles, furans, and thiophenes, with commercially available CF3CH2I as an alkylation reagent. This robust catalytic procedure is scalable and tolerates a broad range of functional groups. Moreover, multifluoroalkylation of indoles is also viable.
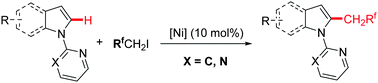


 Please wait while we load your content...
Please wait while we load your content...