Evidence and isolation of tetrahedral intermediates formed upon the addition of lithium carbenoids to Weinreb amides and N-acylpyrroles†
Abstract
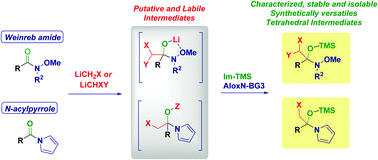
The tetrahedral intermediates generated upon the addition of halolithium carbenoids (LiCH2X and LiCHXY) to Weinreb amides have been intercepted and fully characterized as O-TMS heminals. The commercially available N-trimethylsilyl imidazole is the ideal trapping agent whose employment, combined with a straightforward neutral Alox chromatographic purification, enables the isolation of such labile species. The procedure could be advantageously extended also for obtaining O-TMS heminals from N-acylpyrroles. These intermediates manifest interesting reactivity including as precursors of more complex carbenoids.


 Please wait while we load your content...
Please wait while we load your content...