An atypical interaction explains the high-affinity of a non-hydrolyzable S-linked 1,6-α-mannanase inhibitor†
Abstract
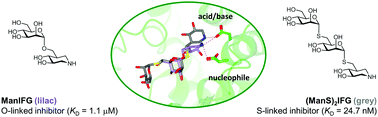
The non-hydrolyzable S-linked azasugars, 1,6-α-mannosylthio- and 1,6-α-mannobiosylthioisofagomine, were synthesized and shown to bind with high affinity to a family 76 endo-1,6-α-mannanase from Bacillus circulans. X-ray crystallography showed an atypical interaction of the isofagomine nitrogen with the catalytic acid/base. Molecular dynamics simulations reveal that the atypical binding results from sulfur perturbing the most stable form away from the nucleophile interaction preferred for the O-linked congener.


 Please wait while we load your content...
Please wait while we load your content...