Metal-free intermolecular cyclopropanation between alkenes and iodonium ylides mediated by PhI(OAc)2·Bu4NI†
Abstract
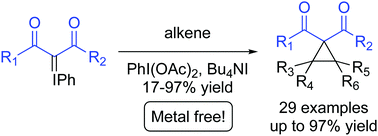
A rapid, mild and metal-free intermolecular cyclopropanation between iodonium ylides and alkene-containing substrates mediated by PhI(OAc)2·Bu4NI is reported. Iodonium ylides of cyclic and acyclic 1,3-dicarbonyls were reacted with a variety of mono-, di-, tri- and tetra-substituted alkenes of various structural types to give 29 cyclopropanes in up to 97% yield.


 Please wait while we load your content...
Please wait while we load your content...