A DFT mechanistic study of the organocatalytic asymmetric reaction of aldehydes and homophthalic anhydride†
Abstract
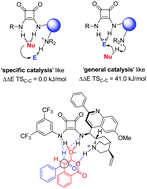
The first DFT study of the cycloaddition of benzaldehyde with homophthalic anhydride under the influence of a bifunctional organocatalyst is reported. The catalyst first binds and then deprotonates the anhydride, leading to a squaramide-bound enolate, which then adds to the aldehyde with activation of the electrophile by the catalyst's ammonium ion.


 Please wait while we load your content...
Please wait while we load your content...