Incorporation of native antibodies and Fc-fusion proteins on DNA nanostructures via a modular conjugation strategy†
Abstract
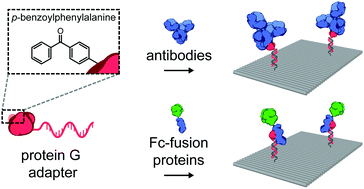
A photocrosslinkable protein G variant was used as an adapter protein to covalently and site-specifically conjugate an antibody and an Fc-fusion protein to an oligonucleotide. This modular approach enables straightforward decoration of DNA nanostructures with complex native proteins while retaining their innate binding affinity, allowing precise control over the nanoscale spatial organization of such proteins for in vitro and in vivo biomedical applications.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...