Facile preparation of pyrimidinediones and thioacrylamides via reductive functionalization of amides†
Abstract
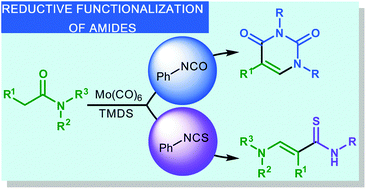
The development of an efficient protocol for the reductive functionalization of amides into pyrimidinediones and amino-substituted thioacrylamides is presented. Enamines are generated in a highly chemoselective amide hydrosilylation reaction catalyzed by molybdenum hexacarbonyl in combination with 1,1,3,3-tetramethyldisiloxane. The direct addition of either isocyanate or isothiocyanate generates the corresponding pyrimidinediones and 3-aminothioacrylamides in high yields.


 Please wait while we load your content...
Please wait while we load your content...