Stereospecific control of the helical orientation of indolocarbazole–pyridine hybrid foldamers by rational modification of terminal chiral appendages†
Abstract
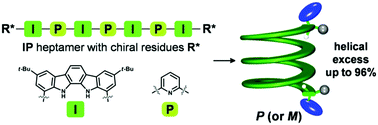
The helical handedness excess of an indolocarbazole–pyridine hybrid oligomer capable of folding into a stable helical structure was achieved up to 96% by rational modification of terminal chiral residues.


 Please wait while we load your content...
Please wait while we load your content...