Stabilizing amyloid-β peptide by the N-terminus capture is capable of preventing and eliminating amyloid-β oligomers†
Abstract
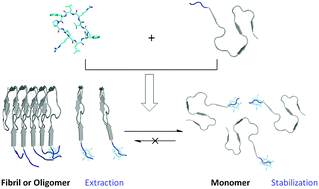
Elimination of amyloid-β (Aβ) oligomers remains challenging. We describe here a novel strategy to prevent and eliminate the Aβ oligomers from either the early aggregation or the fibril dissolution pathway by targeting the flexible N-terminus, but not the widely investigated hydrophobic segment, with a rationally designed cyclopeptide.


 Please wait while we load your content...
Please wait while we load your content...