Catalytic enantioselective aza-Diels–Alder reactions of unactivated acyclic 1,3-dienes with aryl-, alkenyl-, and alkyl-substituted imines†
Abstract
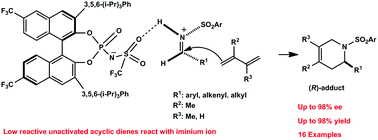
A catalytic enantioselective aza-Diels–Alder reaction of unactivated acyclic dienes with aryl-, alkenyl-, and alkyl-substituted imines is described. With 5–10 mol% loadings of a new Brønsted acid catalyst, the aza-Diels–Alder reaction of unactivated acyclic dienes proceeded to give the corresponding aza-Diels–Alder adducts in high yields (up to 98%) with excellent enantioselectivity (up to 98% ee). Preliminary DFT calculations suggest that the reaction proceeds through a chiral ion pair intermediate.


 Please wait while we load your content...
Please wait while we load your content...