Separating lanthanides and actinides from nitric acid solutions by using N,N-di(2-ethylhexyl)-diglycolamic acid (HDEHDGA)†
Abstract
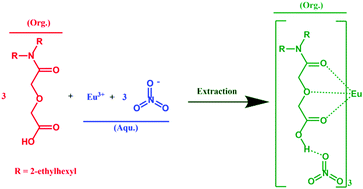
The extraction of Eu(III), U(VI), and Pu(IV) from 0.1–3 M HNO3 solutions by using N,N-di(2-ethyl-hexyl)-diglycolamic acid (HDEHDGA) is presented. In contrast to common organic acid extractants, such as long-chain carboxylic acids, organophosphorus acids, and derivatives of beta-diketones, losing extractability, HDEHDGA exhibits a strong ability to extract lanthanide and actinide ions from high HNO3 solutions.


 Please wait while we load your content...
Please wait while we load your content...