Enantioselective synthesis of pyrazolone α-aminonitrile derivatives via an organocatalytic Strecker reaction†
Abstract
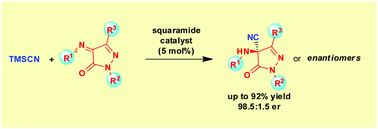
A new organocatalytic enantioselective Strecker reaction of pyrazolone-derived ketimine electrophiles has been developed. Using pseudo-enantiomeric squaramide catalysts the nucleophilic 1,2-addition of trimethylsilyl cyanide to the ketimines efficiently provides a direct entry to both enantiomers of pyrazolone α-aminonitrile derivatives at will in good yields and high enantioselectivities for a wide variety of substrates.


 Please wait while we load your content...
Please wait while we load your content...