Enabling iron catalyzed Doyle–Kirmse rearrangement reactions with in situ generated diazo compounds†
Abstract
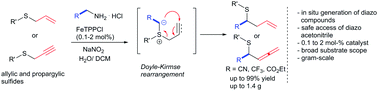
Slow addition of sodium nitrite allows the in situ preparation of highly explosive diazo compounds and enables their safe and scalable application in iron catalyzed rearrangement reactions of allylic and propargylic sulfides. With catalyst loadings as low as 0.1 mol% an effective entry into α-mercapto-nitriles, α-mercapto-esters and α-trifluoromethyl-sulfides on a gram-scale is achieved.


 Please wait while we load your content...
Please wait while we load your content...