Palladium-catalysed atom-economical synthesis of conjugated dienals from terminal acetylenes and acrolein†
Abstract
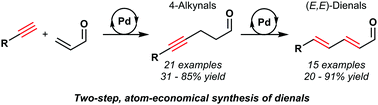
Conjugated (E,E)-dienals are versatile synthetic intermediates owing to their trifunctional, electrophilic nature and the prevalence of the (E,E)-diene in a wide range of functional molecules. It is shown herein that (E,E)-dienals can be readily prepared in two palladium-catalysed steps from simple, unactivated starting materials; terminal acetylenes and acrolein can be coupled via conjugate addition, followed by alkyne isomerisation. This procedure provides a highly atom-economical, redox-neutral and practical method to prepare a range of conjugated (E,E)-dienals in good yields and diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...