An unexpected rearrangement of pyrazolium halides based on N–N bond cleavage: synthesis of 1,2-dihydropyrimidines†
Abstract
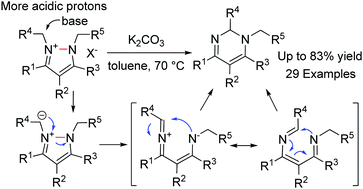
The rearrangement of pyrazolium halides was observed, giving 1,2-dihydropyrimidines in good to high yields. A broad scope of pyrazolium halides bearing various N-substituted benzyls or alkyls can be utilized. A mechanistic study using isotope labelling experiments suggested that the present rearrangement proceeds through an ylide formation/ring cleavage/ring closure pathway to yield the observed product.


 Please wait while we load your content...
Please wait while we load your content...