Two cations, two mechanisms: interactions of sodium and calcium with zwitterionic lipid membranes†
Abstract
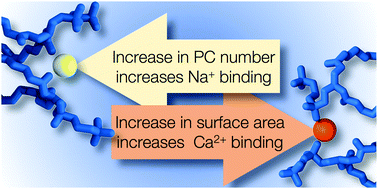
Adsorption of metal cations onto a cellular membrane changes its properties, such as interactions with charged moieties or the propensity for membrane fusion. It is, however, unclear whether cells can regulate ion adsorption and the related functions via locally adjusting their membrane composition. We employed fluorescence techniques and computer simulations to determine how the presence of cholesterol—a key molecule inducing membrane heterogeneity—affects the adsorption of sodium and calcium onto zwitterionic phosphatidylcholine bilayers. We found that the transient adsorption of sodium is dependent on the number of phosphatidylcholine head groups, while the strong surface binding of calcium is determined by the available surface area of the membrane. Cholesterol thus does not affect sodium adsorption and only plays an indirect role in modulating the adsorption of calcium by increasing the total surface area of the membrane. These observations also indicate how lateral lipid heterogeneity can regulate various ion-induced processes including adsorption of peripheral proteins, nanoparticles, and other molecules onto membranes.


 Please wait while we load your content...
Please wait while we load your content...