KOtBu-Catalyzed lithiation of PMDTA and the direct functionalization of bridged alkenes under mild conditions†
Abstract
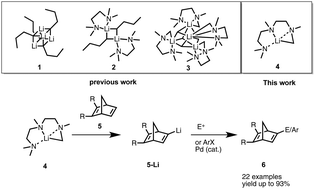
A practical preparation of the reagent PMDTALi using a super base system under mild conditions has been developed. This PMDTALi base has been demonstrated to be a very efficient reagent for the lithiation of bridged alkenes via direct deprotonation. Further reactions with electrophiles and also coupling reactions in the presence of Pd catalysts provide the bridged alkenes with a broad range of functional groups including silyl, alkyl, halogen and aryl substituents. The utilization of this new lithium reagent has brought a new diversity to the choice of lithium reagent for the deprotonation of synthetically challenging systems.


 Please wait while we load your content...
Please wait while we load your content...