Regioselective copper-catalyzed direct arylation of benzodithiophene-S,S-tetraoxide†
Abstract
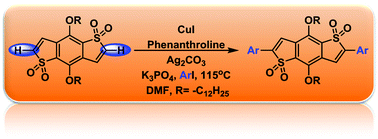
An efficient copper-catalyzed direct arylation reaction for the regioselective functionalization of benzodithiophene-S,S-tetraoxide has been developed. The method demonstrates a broad scope with isolated yields ranging from good to excellent. Furthermore, the reaction specificity for aryl iodides over the unreactive aryl bromides provide a opportunity to generate a new donor–acceptor–donor triad.


 Please wait while we load your content...
Please wait while we load your content...