Multinuclear zinc bisamidinate catalyzed asymmetric alkylation of α-ketoesters and its unique chemoselectivity†
Abstract
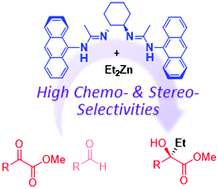
The multinuclear Zn-bisamidinate catalyzed enantioselective addition of Et2Zn to α-ketoesters has been developed. The steric tuning of two amidinate units as well as multiple coordination on the Zn atoms play a key role in achieving high enantioselectivity (up to 98% ee) and unique chemoselectivity. The present catalyst exhibited the preferential alkylation of α-ketoesters even in the presence of aldehydes.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...