Rearrangement of an aniline linked perylene bisimide under acidic conditions and visible to near-infrared emission from the intramolecular charge-transfer state of its fused derivatives†
Abstract
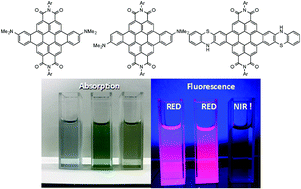
We have prepared a series of aniline-linked and fused perylene bisimides (PBIs) for making near-infrared (NIR) fluorophores. During this research, we found an unexpected rearrangement reaction on the PBI core for the first time. The aniline- and phenothiazine-fused PBIs exhibit excellent absorption ability and visible-to-NIR emission owing to their intramolecular charge transfer character.


 Please wait while we load your content...
Please wait while we load your content...