Controlled ring-opening polymerization of α-amino acid N-carboxy-anhydride by frustrated amine/borane Lewis pairs†
Abstract
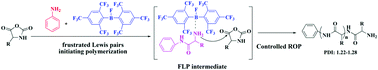
In this communication, we presented a novel strategy to control the ROP of α-amino acid N-carboxy-anhydrides using the concept of frustrated Lewis pairs (FLPs). An FLP intermediate containing an interaction between the bulky borane Lewis acid and the amine groups of the propagation chain end is essential to accomplish the polypeptide synthesis with well-defined structures under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...