Asymmetric hydroarylation of vinyl ethers catalyzed by a hydroxoiridium complex: azoles as effective directing groups†
Abstract
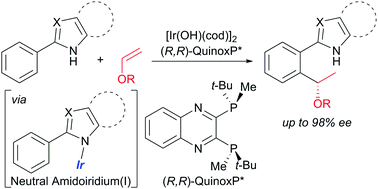
Catalytic asymmetric hydroarylation of vinyl ethers using 2-aryl-substituted azoles containing an N–H bond as directing groups was realized by use of a hydroxoiridium/chiral phosphine catalyst. The reaction proceeded via C–H activation of the aromatic ring to give high yields of the corresponding addition products with high branch- and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...