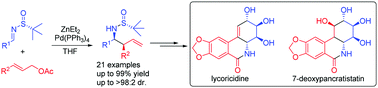
Asymmetric cinnamylation of N-tert-butanesulfinyl imines with cinnamyl acetates: total syntheses of (+)-lycoricidine and (+)-7-deoxypancratistatin†
Abstract
Highly diastereoselective palladium catalyzed cinnamylation of N-tert-butanesulfinyl imines with cinnamyl acetates has been established to provide enantioenriched β-aryl homoallylic amines. The synthetic application of this stragety has been successfully demonstrated in the concise total syntheses of antitumor natural products (+)-lycoricidine and (+)-7-deoxypancratistatin.


 Please wait while we load your content...
Please wait while we load your content...