Mechanistic investigation of the NH-sulfoximination of sulfide. Evidence for λ6-sulfanenitrile intermediates†
Abstract
We report a new procedure for the preparation of NH-sulfoximines from sulfides using PIDA as an oxidant and ammonium carbamate as the ammonia source. Excellent yields were obtained with a wide range of sulfides. The formation of acetoxy- and methoxy-λ6-sulfanenitrile as intermediates was proposed, both of which were converted to the NH-sulfoximine by the action of the solvent. The structure of these intermediates was confirmed by 1H, 13C and 15N NMR and HRMS analysis.
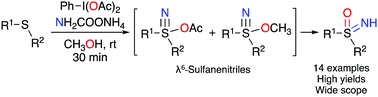


 Please wait while we load your content...
Please wait while we load your content...