Ambiguous reactivity of Li/Cl phosphinidenoid complexes under redox conditions – a novel dichotomy in phosphorus chemistry†
Abstract
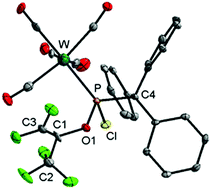
A novel ambiguous reactivity of Li/Cl phosphinidenoid complexes under redox conditions is described. The outcome of the reaction with hexafluoroacetone is highly dependent on the P-substituent as fluoride substitution occurred in the case of R = CPh3 and C5Me5via a radical pathway, whereas for R = CH(SiMe3)2 a complex having a novel 1,2-diol-type P-ligand was obtained via a closed-shell pathway. DFT calculations reveal a new SET pathway starting with a noncovalent π-hole complex between the phosphinidenoid anion and hexafluoroacetone followed by an elimination of LiF. The second, closed-shell reaction course is strongly influenced by a noncovalent O⋯Si interaction established after the initial nucleophilic attack.


 Please wait while we load your content...
Please wait while we load your content...