Three-component carboarylation of unactivated imines with arynes and carbon nucleophiles†
Abstract
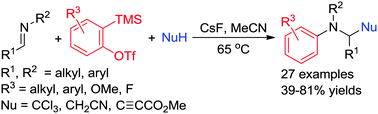
With 2-(trimethylsilyl)aryl triflates as aryne precursors, an unprecedented three-component carboarylation reaction of unactivated imines with arynes and carbon nucleophiles has been developed to access a variety of functionalized tertiary amines under transition metal-free conditions. Suitable carbon nucleophiles include chloroform, acetonitrile, and methyl propiolate.


 Please wait while we load your content...
Please wait while we load your content...