Lowering water oxidation overpotentials using the ionisable imidazole of copper(2-(2′-pyridyl)imidazole)†
Abstract
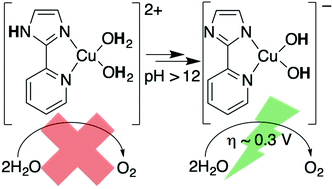
Rapid and low overpotential oxidation of water to dioxygen remains a key hurdle for storage of solar energy. Here, we address this issue by demonstrating that deprotonation of 2-(2′-pyridyl)-imidazole (pimH)-ligated copper complexes promotes water oxidation at low overpotential and low catalyst loading. This improves upon other work on homogeneous copper-based water oxidation catalysts, which are highly active, but limited by high overpotentials. EPR and UV-vis spectroscopic evaluation of catalyst speciation shows that at pH ≥ 12 coordinated pimH is deprotonated and a bis(hydroxide) Cu2+ active catalyst forms. Rapid electrochemical water oxidation (35 s−1, 0.85 V onset potential) was observed with 150 μM catalyst. These results demonstrate that catalytic water oxidation potentials can be shifted by hundreds of mV in homogeneous metal catalysts bearing an ionisable imidazole ligand.


 Please wait while we load your content...
Please wait while we load your content...