Direct thermolysis of CO2 into CO and O2†
Abstract
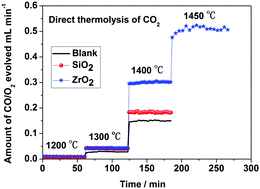
Most of the solar-driven thermochemical CO2 dissociation reactions have been focused on two-step processes. In this study, a one step CO2 thermolysis process was considered. It was found that direct thermolysis of CO2 obviously occurred at temperatures as low as 1200 °C within a corundum tube. The reaction rate could be enhanced by several times in the presence of metal oxides, which may be attributed to the catalysis of oxygen vacancies in the metal oxides.


 Please wait while we load your content...
Please wait while we load your content...