(+)-Camphor-mediated kinetic resolution of allylalanes: a strategy towards enantio-enriched cyclohex-2-en-1-ylalane†
Abstract
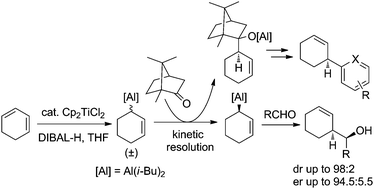
An efficient (+)-camphor-mediated kinetic resolution of racemic cyclohex-2-en-1-ylalane is described. This approach provides an enantiomerically enriched form of the alane, in situ available for synthetic uses. Applied to the allylation of aldehydes, this protocol leads to the corresponding homoallylalcohols in a highly enantioselective manner.

 Please wait while we load your content...
Please wait while we load your content...