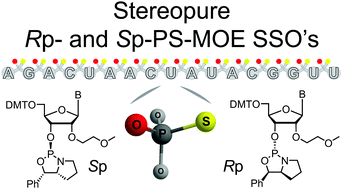
Synthesis and cellular activity of stereochemically-pure 2′-O-(2-methoxyethyl)-phosphorothioate oligonucleotides†
Abstract
Stereochemically-pure 2′-O-(2-methoxyethyl)-phosphorothioate (PS-MOE) oligonucleotides were synthesized from new chiral oxazaphospholidine-containing nucleosides. Thermal stability studies showed that the incorporation of Rp-PS linkages increased RNA-binding affinity. In cells, a full Rp-PS-MOE splice-switching oligonucleotide targeting part of the ferrochelatase gene was more potent than its Sp-PS counterpart, but of similar potency to the stereorandom PS-parent sequence.


 Please wait while we load your content...
Please wait while we load your content...