Facile synthesis of cyanofurans via Michael-addition/cyclization of ene–yne–ketones with trimethylsilyl cyanide†
Abstract
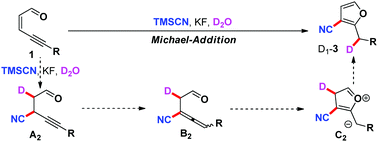
We have developed a Michael-addition/cyclization procedure between ene–yne–ketones and TMSCN under metal-free conditions. A wide range of cyanofurans was delivered in high yields, which could be further transformed to a series of furo-furanimines, furo-pyridazines or carboxamido-furans. In addition, deuterium-labeling experiments have been conducted to clarify the reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...