From the configurational preference of dihydroceramide desaturase-1 towards Δ6-unsaturated substrates to the discovery of a new inhibitor†
Abstract
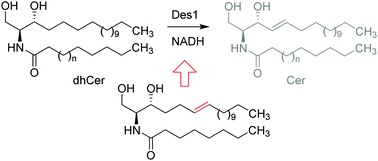
Dihydroceramide desaturase 1 (Des1) catalyzes the last step of ceramide synthesis de novo, thus regulating the physiologically relevant balance between dihydrosphingolipids and sphingolipids. Here we report on the configurational preference of Des1 towards isomeric Δ6-unsaturated dihydroceramide analogs and the discovery of a potent Des1 inhibitor.


 Please wait while we load your content...
Please wait while we load your content...