Effect of the lipid II sugar moiety on bacterial transglycosylase: the 4-hydroxy epimer of lipid II is a TGase inhibitor†
Abstract
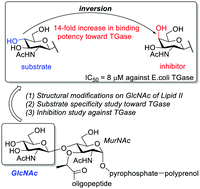
Lipid II analogues bearing major modifications on the second sugar (GlcNAc) were synthesized and evaluated for their substrate activity toward TGases. Unexpectedly, N-deacetyled lipid II decreased its activity dramatically, and the C4-axial OH lipid II became an inhibitor (IC50 = 8 μM) with an approximately 14-fold increase in binding affinity toward TGase (25 vs. 27).


 Please wait while we load your content...
Please wait while we load your content...