Chemistry at the square nanometer: reactivity at liquid/solid interfaces revealed with an STM
Abstract
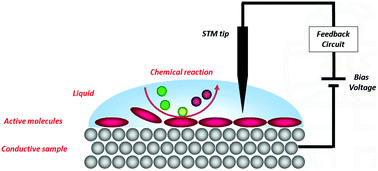
For more than three decades the scanning tunnelling microscope (STM) has proven to be an indispensable tool to image molecules adsorbed at a surface at the highest detail possible. In addition to simply imaging molecules, STM can also be applied to monitor dynamic surface phenomena, including chemical reactions. By studying reactions at a surface at the single molecule level, unique information about reaction mechanisms can be obtained which remains hidden when conventional ensemble techniques are used. Many STM studies of chemical reactions have been performed in extreme environments like ultrahigh vacuum or high pressure chambers, but these are far removed from conditions in which most chemical and biological processes take place, i.e., in a liquid at ambient atmospheres. This feature paper highlights the developments in the relatively unexplored research area of investigating chemical reactions with an STM at a liquid/solid interface under ambient conditions. Covalent couplings between molecules, light-induced isomerisations, reactions under electrochemical control, and complex multistep processes and catalysis are discussed.


 Please wait while we load your content...
Please wait while we load your content...