Benzimidazopurine nucleosides from N6-aryl adenosine derivatives by PhI(OAc)2-mediated C–N bond formation, no metal needed†
Abstract
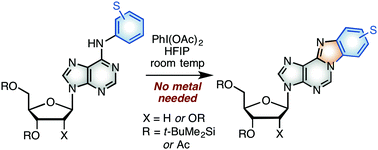
The reaction of a variety of N6-aryl 2′-deoxyadenosine and adenosine derivatives with PhI(OAc)2 in 1,1,1,3,3,3-hexafluoro-2-propanol provides a facile access to benzimidazopurine nucleoside analogues by metal-free C–N bond formation with a purinyl nitrogen atom. These reactions likely proceed via radical-cation/radical processes as indicated by radical inhibition experiments.


 Please wait while we load your content...
Please wait while we load your content...